OPIOID OVERDOSE CRISIS
Opioid overdoses are full of unknowns. The variability and potency of opioids, particularly synthetics, make reversal situations even more challenging.2,3
Actor portrayals.
Fentanyl and other synthetics (except methadone) increased opioid overdose deaths 7.5-fold from 2015–2021.
In 2021, this number increased to 70,601 overdose deaths—mostly from fentanyl.
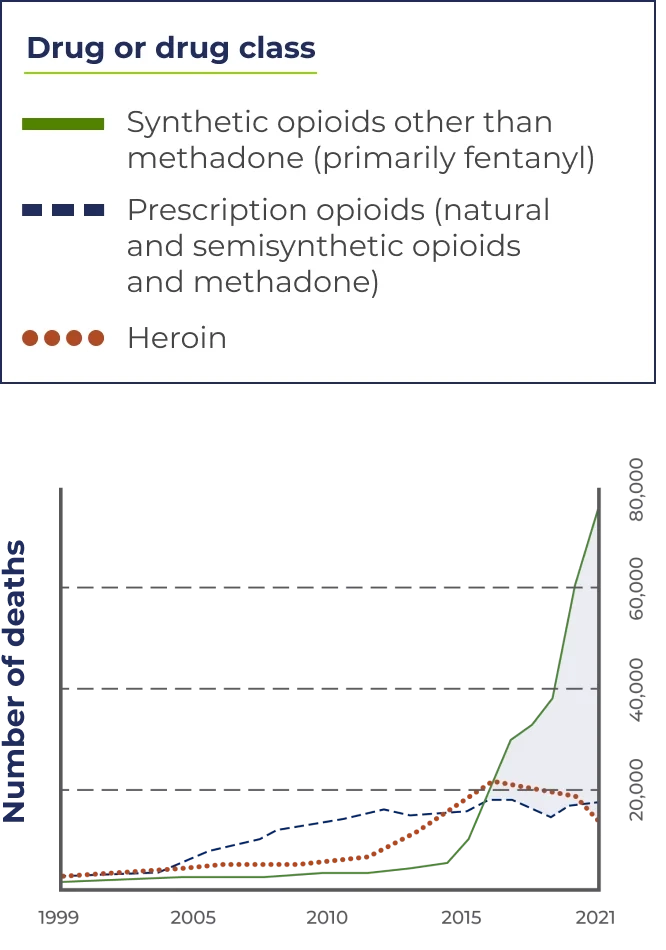
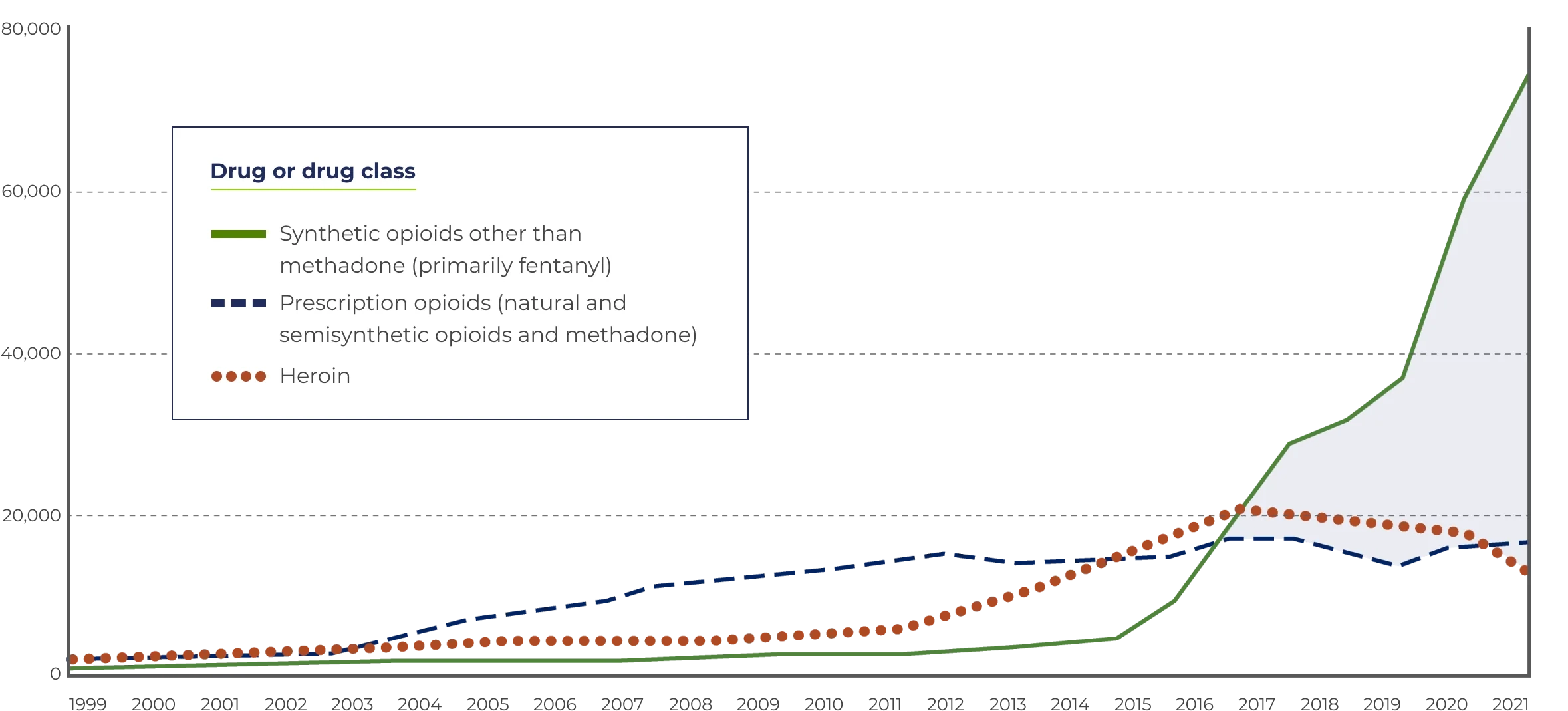
*Includes deaths with underlying causes of unintentional drug poisoning (X40–X44), suicide drug poisoning (X60–X64), homicide drug poisoning (X85), or drug poisoning of undetermined intent (Y10–Y14), as coded in the International Classification of Diseases, 10th Revision.
Source: Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999–2021. CDC WONDER Online Database, 01/23.
The National Institutes of Health (NIH) supports development of stronger, longer-acting formulations of opioid antagonists to counteract high-potency synthetic opioids.5

| Opioids | Drug | Plasma half-life (hours) |
|---|---|---|
Natural | Morphine6 | 3 to 4 |
Semisynthetic | Heroin7 | 0.5 |
Hydromorphone6 | 2 to 3 | |
Oxycodone8 | 3 to 5 | |
Synthetic | Fentanyl6, 9-11 | |
Carfentanil12 | ||
Tramadol13 | ||
Methadone6 |
†This is not an exhaustive list of all opioids. Half-lives reported are approximations. Patient- and drug-specific variables may impact the actual half-life of a drug. Pharmacokinetic data may not necessarily correlate with clinical effects.
| Drug | Elimination mean plasma half-life (t1/2) | Route of administration Route of admin-istration |
|---|---|---|
Nalmefene HCl1injection | 10.8 hours§ | IV, IM, or SC |
Naloxone HCl (generic)14injection | 0.5–1.35 hours | |
ZIMHI® | IM, 1.5 hours | IM or SC |
OPVEE® (nalmefene)16nasal spray | 11.4 hours | IN |
NARCAN® (naloxone HCl)17nasal spray | Approximately 2.08 hours | |
Kloxxado® (naloxone HCl)18nasal spray | 1.8–2.7 hours | |
RiVive™ | 1.36 hours |
IM=intramuscular; IN=intranasal; IV=intravenous; SC=subcutaneous.
‡Not an exhaustive list of all half-lives reported across all studies conducted. Based on values reported in each respective full Prescribing Information and New Drug Application.
§Nalmefene elimination half-life is based on pharmacokinetic data. The mean terminal elimination half-life is 10.8 hours and 9.4 hours in
IM=intramuscular; IV=intravenous; SC=subcutaneous.
Studied in 284 patients presumed to have taken an opioid overdose. Effectively reversed respiratory depression at doses of 0.5 mg to 1 mg. Dose range in clinical trials was 0.5 mg to 2 mg.1,20
Nalmefene elimination half-life is based on pharmacokinetic data. The mean terminal elimination half-life is 10.8 hours and 9.4 hours in younger (19 to 32 years) and elderly (62 to 80 years) healthy male subjects, respectively, following a Nalmefene HCl 1 mg IV dose.1